Seawater Is Typically Denser Than Freshwater Due to Seawater's
The freezing point of seawater. Seawater is denser than fresh water but not all seawater is of equal density.

Earth Science 2007 Released Test 1 Why Does A Comet S Tail Point Away From The Sun The Solar Wind Blows The Tail Away From The Sun 2 It Is Ppt Download
Saltwater intrusion can naturally occur in coastal aquifers owing to the hydraulic connection between groundwater and seawaterBecause saline water has a higher mineral content than.

. Sea water contains dissolved minerals and salts in it due to which it has high densityThe density of seawater varies with temperature and salinity of the water. Density of sea water is more than density of river water. How Is Ocean Water Different From Freshwater.
The more dense water will float and the less dense water will sink. Ice is less dense than water because. Freshwater freezes at zero degrees Celsius.
This makes the density. Thus colder fresh water is less dense which has implications for lake overturn and ice floating As salinity is increased the density maximum moves to lower temperature. Typically the salinity decreases from the surface ocean to deep waters is very small from about 36 gL ppt at the surface to 35 gL ppt in the deep water thus there is a very small density decrease with depth given a constant temperature.
The freezing point of seawater decreases as salt concentration increases. Salinity influences this relationship in that the saltier the water the denser it becomes. Due to the lack of salt content in fresh water.
Seawater is denser than both fresh water and pure water density 10 kgl at 4 C 39 F because the dissolved salts increase the mass by a larger proportion than the volume. However seawater contains many different salts and minerals. The density of seawater ranges from 1020 to 1030 kgm 3 while the density of freshwater is about 1000 kgm 3.
This is because the dissolved salt makes seawater denser. The temperature and salinity of the sea water also help determine its density. Everything else being equivalent a saline water Suiton projectile will therefore deal more damage than a fresh water one.
How do temperature and salinity affect seawater density quizlet. If I have freshwater that is less dense what will it do in comparison to salt water that is more dense. Water molecules in ice crystals take up more space than water molecules.
This density also means higher buoyancy. True Equatorial regions low latitudes often have little to no layering of the water as the thermoclines and pycnoclines are typically absent or poorly developed. Average density at the surface is 1025 kgl.
Since salt ions are heavier than water molecules seawater is denser than freshwater. Average density at the surface is 1025 kgl. Also as the salt content of sea water increases so does its density.
The density of seawater can vary and is affected by two primary factors. These differences in density help drive under-water currents which circulate ocean water around the globe. As salinity of the water increases density also increases.
Furthermore salinity lowers the freezing point to below 0C 32F. Seawater is denser than both fresh water and pure water density 10 kgl at 4 C 39 F because the dissolved salts increase the mass by a larger proportion than the volume. Seawater is slightly denser than pure 0ppt water.
Saltwater intrusion is the movement of saline water into freshwater aquifers which can lead to groundwater quality degradation including drinking water sources and other consequences. This resulting seawater is denser than freshwater because of added mass of dissolved salt. For pure water density increases as the temperature decreases.
Answer choices The more dense water will float and the less dense water will sink. Seawater is denser than both fresh water and pure water density 10 kgl at 4 C 39 F because the dissolved salts increase the mass by a larger proportion than the volume. Students think that seawater contains table salt sodium chloride.
Variations in salinity also cause the freezing point of seawater to be somewhat lower than that of freshwater. As the temperature of sea water decreases the density also increases. When it rains the freshwater reduces the saltiness of the saltwater on the surface.
The freezing point of seawater. Density as a function of temperature for pure and salty water. Fresh water S0 at atmospheric pressure p0 has maximum density at temperature 4C.
Seawater is denser than fresh water due to the sodium chloride dissolved in it. As temperature increases density decreases. The more dense water will sink and the less dense.
Saltwater is denser than fresh water because of its salt content. Water exists in all three phases solid liquid and vapor on Earths surface because of the presence of ___________ bonds. The salinity of ocean water makes.
If I have freshwater that is less dense what will it do in comparison to salt water that is more dense.

Unit 1 Oceanography Other Quiz Quizizz

Cement And Oil Refining Industries As The Predominant Sources Of Trace Metal Pollution In The Red Sea A Systematic Study Of Element Concentrations In The Red Sea Zooplankton Sciencedirect
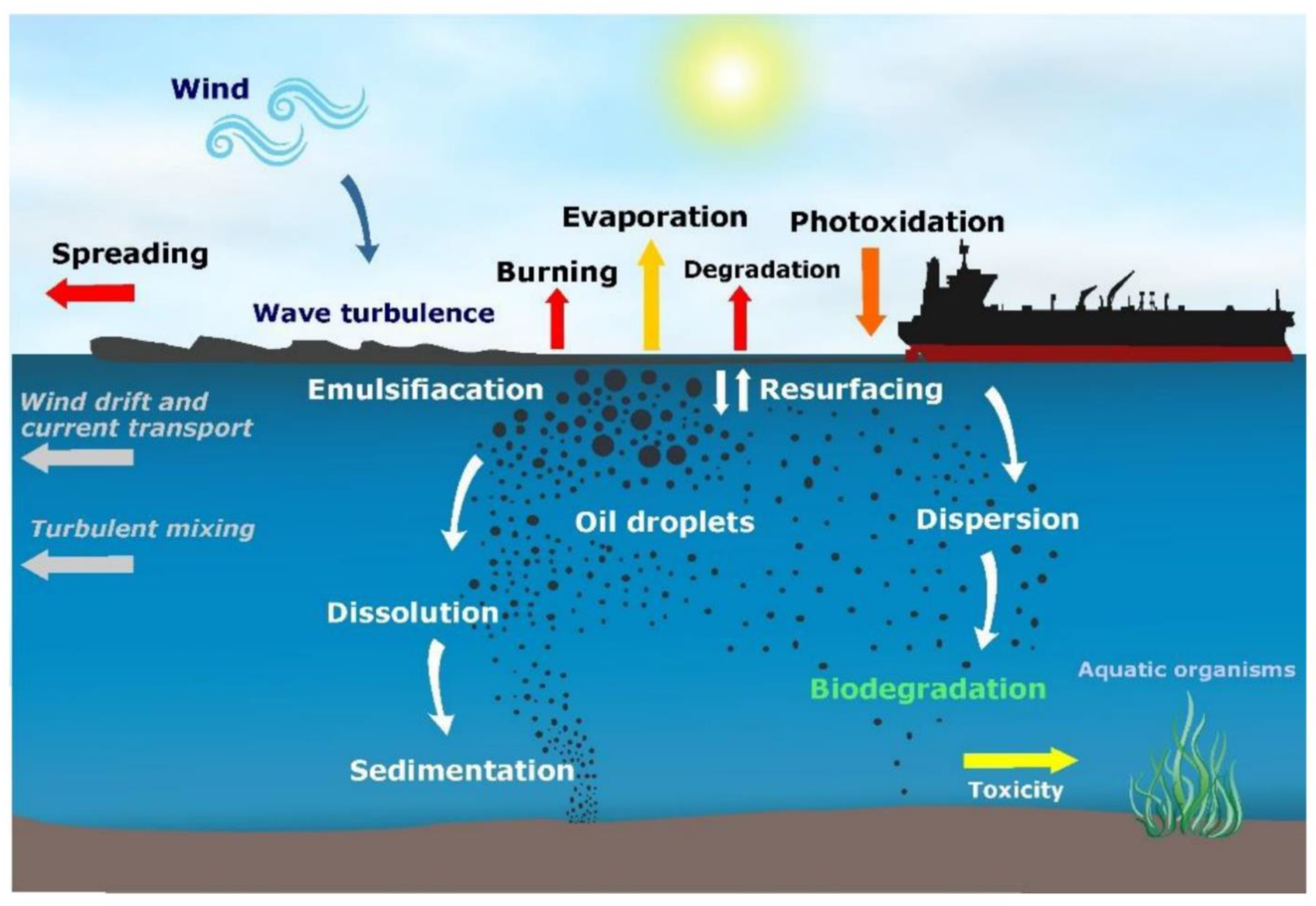
Jmse Free Full Text From Surface Water To The Deep Sea A Review On Factors Affecting The Biodegradation Of Spilled Oil In Marine Environment Html

Sol Exam Questions On Oceanography Fresh Water Ppt Video Online Download

Oceanography Earth Sciences Quiz Quizizz

Who Wants To Be A Millionaire Ppt Download

Properties Of Ocean Water 5th Per Quiz Quizizz

Who Wants To Be A Millionaire Ppt Download

Final Exam Review June Ppt Video Online Download

Who Wants To Be A Millionaire Ppt Download

Sol Exam Questions On Oceanography Fresh Water Ppt Video Online Download

Pdf Teaching Physical Concepts In Oceanography An Inquiry Based Approach





Comments
Post a Comment